Rheumatoid Arthritis and Chronic Inflammation Management: Can Medical Food Like ACEND Offer Clinically Relevant Support?
August 7, 2025
Contributing Authors: Team TRILITY / ACEND
Rheumatoid Arthritis (RA) is a debilitating autoimmune disease that affects over 1.3 million Americans and more than 18 million individuals worldwide. Characterized by chronic inflammation of the synovial joints, RA is driven by a cascade of immune dysfunction, oxidative stress, and systemic inflammation that extends far beyond the joints.
While disease-modifying antirheumatic drugs (DMARDs) and biologics remain standard of care, many patients seek complementary therapies that target the root causes of chronic inflammation without the side effect burden associated with pharmaceuticals. Increasingly, clinicians and researchers are turning to precision nutrition and clinically formulated medical foods to fill this critical gap.
ACEND, a next-generation medical food developed to target chronic inflammation at its source, may offer multi-mechanistic support for individuals living with RA. In this article, we explore the clinical rationale for its use, drawing from the latest scientific literature—including findings from the MDPI review, “Nutritional Modulation of Inflammation in Rheumatoid Arthritis” (2024) .
RA Pathophysiology: A Complex Interplay of Immunity and Inflammation
RA involves the hyperactivation of both the innate and adaptive immune systems. Key drivers include:
-
Pro-inflammatory cytokines: TNF-α, IL-1β, IL-6, IL-17
-
Immune cell dysregulation: Th17/Treg imbalance, overactive macrophages
-
Oxidative stress: Elevated ROS damages cartilage and bone
-
Gut microbiota disruption: Dysbiosis fuels systemic inflammation
These inflammatory mediators not only cause synovial hyperplasia and joint destruction but also contribute to extra-articular manifestations in the cardiovascular, pulmonary, and gastrointestinal systems.
Targeting these inflammatory pathways with nutritional precision—without suppressing immune surveillance—is a promising adjunctive strategy.
ACEND may help target underlying causes of RA
ACEND: A Clinically Formulated Medical Food for Chronic Inflammation
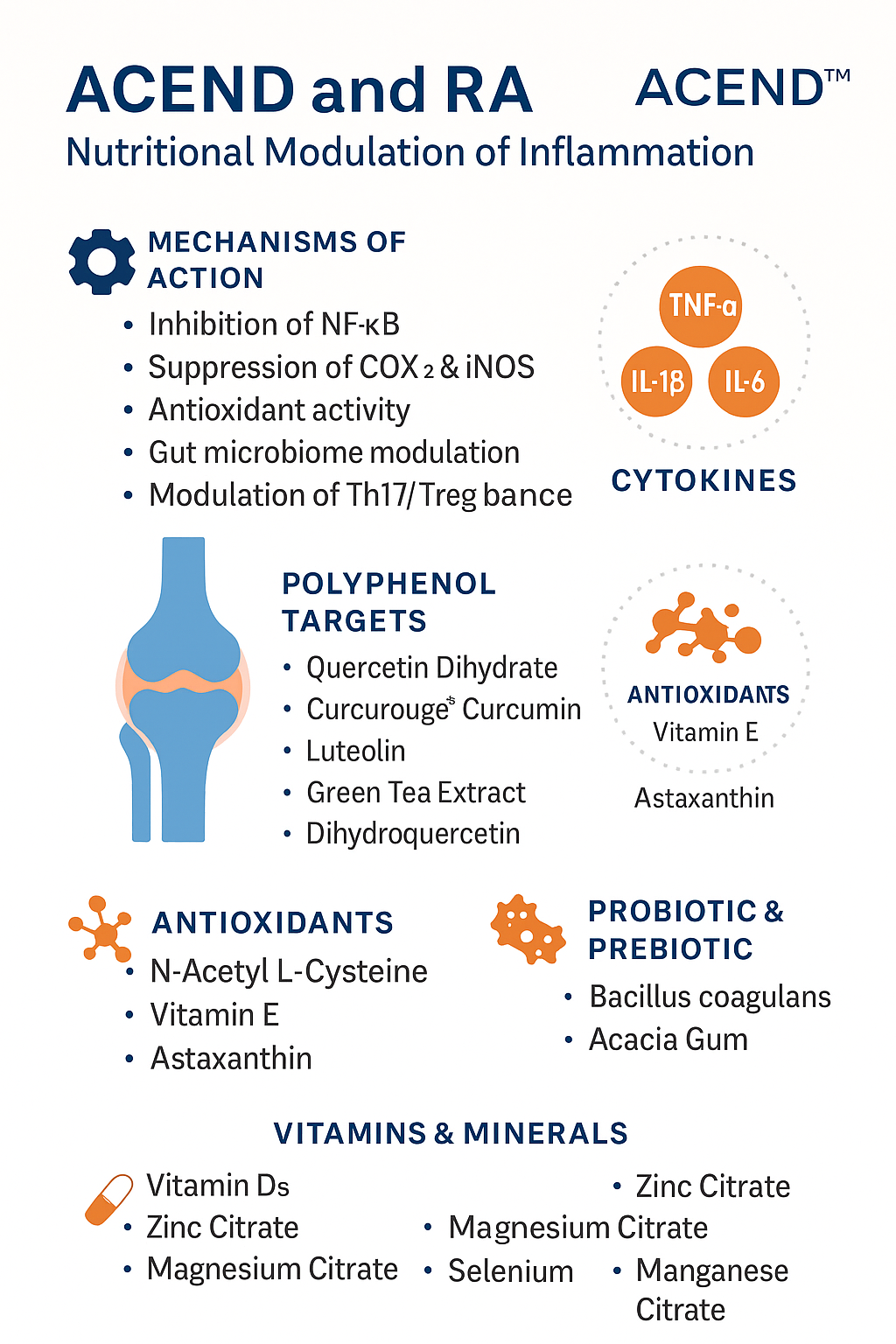
ACEND is not a supplement. It is a medical food, developed to support the management of inflammation-related conditions through scientifically validated bioactives, polyphenols, minerals, vitamins, and microbial modulators.
ACEND’s active compounds target:
-
Pro-inflammatory cytokines (TNF-α, IL-6, IL-1β)
-
Oxidative stress pathways (ROS, NF-κB)
-
Gut microbiota composition and barrier function
-
Immune cell signaling (Th17/Treg modulation)
Let’s examine the scientific rationale for ACEND’s application in RA management.
Polyphenols: Natural Cytokine Modulators
The 2024 MDPI review emphasizes that polyphenols exhibit strong anti-inflammatory and immunomodulatory effects in RA by inhibiting NF-κB, COX-2, and MAPK pathways while reducing cytokine production .
Key Polyphenols in ACEND:
| Compound | Mechanisms of Action | RA-Relevant Targets |
|---|---|---|
| Quercetin Dihydrate | Inhibits TNF-α, IL-1β, reduces leukocyte infiltration | Synovial inflammation |
| Curcurouge® Curcumin | Clinically enhanced bioavailability, COX-2 inhibitor, modulates NF-κB and IL-6 | Joint pain and swelling |
| Luteolin (98%) | Inhibits Th17, suppresses IL-17, reduces ROS and MMPs | Cartilage protection |
| Green Tea Extract (90% (-)-Epicatechins) | Blocks NF-κB, suppresses inflammatory cytokines, supports Treg activity | Immune modulation |
| Dihydroquercetin (Taxifolin) | Anti-oxidative, reduces nitric oxide (NO) and iNOS expression | Oxidative joint damage |
| Dihydromyricetin (DHM) | Regulates macrophage polarization, inhibits IL-1β, promotes joint protection | Macrophage-mediated damage |
These are all small molecule polyphenols, enhancing cellular absorption and allowing them to modulate intracellular inflammatory cascades more effectively.
Antioxidants and Inflammatory Damage Control
Oxidative stress is a hallmark of RA pathology. Reactive oxygen species (ROS) damage joint tissues, promote cytokine expression, and perpetuate chronic inflammation.
ACEND Includes:
-
N-Acetyl L-Cysteine (NAC) → Precursor to glutathione, modulates NF-κB, reduces IL-8, IL-1β
-
Vitamin E (d-alpha tocopherol succinate) → Lipid antioxidant that protects synovial membranes
-
Astaxanthin (BioAstin®) → One of the most potent known antioxidants, downregulates COX-2 and protects mitochondria
These compounds help preserve cartilage, reduce mitochondrial dysfunction, and protect against the catabolic effects of prolonged inflammation.
The Gut-Immune Connection in RA
Several studies—including those cited in the MDPI review—indicate that dysbiosis and gut barrier dysfunction play a role in initiating and perpetuating RA .
RA patients often display:
-
Lower diversity of gut microbes
-
Higher abundance of pro-inflammatory strains (e.g., Prevotella copri)
-
Increased intestinal permeability (“leaky gut”)
ACEND’s Gut-Focused Components:
-
LactoSpore® Bacillus coagulans (15 Billion CFU/g)
→ Clinically studied to reduce LPS, increase tight junction proteins, support IL-10 production -
Organic Acacia Gum (prebiotic)
→ Promotes SCFA (butyrate) production, increases Faecalibacterium prausnitzii, an anti-inflammatory commensal
Together, these create a functional gut-immune axis, reducing systemic inflammation driven by microbial endotoxins.
Vitamin D3 and Immune Regulation
Vitamin D deficiency is disproportionately common in RA patients and is associated with:
-
Higher disease activity
-
Increased joint damage
-
Elevated IL-17 and reduced Treg cells
ACEND Includes:
-
Vitamin D3 (Cholecalciferol) at therapeutic doses
Vitamin D3 supports immune tolerance, improves response to conventional therapy, and may reduce flare frequency.
Supportive Minerals and Micronutrients
RA is associated with deficiencies in key trace elements involved in antioxidant defense and immune signaling.
Included in ACEND:
-
Zinc Citrate → Cofactor for over 300 enzymes, essential for T cell modulation
-
Magnesium Citrate → Modulates IL-6 and TNF-α, reduces fatigue and pain
-
Selenium (Selenomethionine) → Boosts glutathione peroxidase, reduces inflammatory burden
-
Manganese, Boron, Chromium → Support bone health, glucose metabolism, and antioxidant function
These minerals are included in bioavailable chelated or citrate forms, maximizing absorption and efficacy.
Why ACEND May Be Uniquely Suited for RA
Many nutraceuticals offer one or two anti-inflammatory compounds. ACEND provides a multi-pronged, systems biology-based formulation that:
-
Targets core inflammatory cytokines
-
Regulates Th17/Treg balance
-
Restores gut microbial health
-
Protects against oxidative joint damage
-
Supports bone and cartilage integrity
Unlike supplements, ACEND is regulated as a medical food, designed for use under physician supervision and supported by scientific rationale for chronic inflammation management.
Clinical Application
For clinicians, ACEND may serve as a safe adjunct to:
-
DMARD therapy
-
Biologic agents
-
Corticosteroid tapering strategies
-
Autoimmune flare reduction protocols
For patients, ACEND offers:
-
Drug-free support for chronic inflammation
-
Gut and immune axis restoration
-
Enhanced antioxidant capacity
-
Reduced flare frequency and fatigue
So, Where Does This Leave Us?
Rheumatoid Arthritis is a multifaceted disease rooted in systemic immune dysregulation and inflammation. While standard medications remain essential, the evidence increasingly supports nutritional intervention as a critical part of disease management.
Based on current literature, the clinical profile of ACEND offers a compelling rationale for its role in comprehensive RA support. Its inclusion of high-bioavailability polyphenols, antioxidant support, microbial modulators, and micronutrients reflects the latest research into the pathophysiology of RA.
Therefore, we suggest that ACEND—when used under healthcare provider supervision—may represent a valuable therapeutic tool in the fight against RA-driven inflammation.
References
-
Di Renzo, L., et al. (2024). Nutritional Modulation of Inflammation in Rheumatoid Arthritis. Nutrients, 17(9), 1603. https://doi.org/10.3390/nu17091603
-
Bao, Y., et al. (2018). Quercetin inhibits TNF-α induced inflammation in human synoviocytes. Inflammation Research, 67, 637–648.
-
Aggarwal, B. B., et al. (2013). Curcumin: The Indian solid gold. Advances in Experimental Medicine and Biology, 595, 1–75.
-
Seelinger, G., et al. (2008). Anti-carcinogenic effects of the flavonoid luteolin. Molecular Nutrition & Food Research, 52(1), 124–134.
-
Jeong, S. Y., et al. (2020). Green tea polyphenol epigallocatechin-3-gallate (EGCG) attenuates rheumatoid arthritis inflammation. Journal of Nutritional Biochemistry, 85, 108450.
-
Zhang, L., et al. (2021). Taxifolin inhibits inflammatory responses by downregulating TLR4 and ROS-NLRP3 pathways. Oxidative Medicine and Cellular Longevity, Article ID 9466482.
-
Zhang, X., et al. (2021). Dihydromyricetin suppresses inflammation via macrophage polarization. Frontiers in Pharmacology, 12, 683046.
-
Rushworth, G. F., & Megson, I. L. (2014). Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione. Clinical Pharmacokinetics, 53(6), 539–552.
-
Higdon, J. V., & Frei, B. (2003). Vitamin E and cardiovascular disease. Atherosclerosis, 161(2), 271–288.
-
Fassett, R. G., & Coombes, J. S. (2011). Astaxanthin: A potential therapeutic agent in cardiovascular disease. Marine Drugs, 9(3), 447–465.
-
Maeda, Y., et al. (2016). Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis & Rheumatology, 68(11), 2646–2661.
-
Sudha, M. R., et al. (2011). Immunomodulatory effect of Bacillus coagulans. Indian Journal of Medical Research, 134, 168–173.
-
Scott, K. P., et al. (2013). Dietary fiber and the gut microbiota. Nutrition Bulletin, 38(3), 298–303.
-
Craig, S. M., & Yu, F. (2010). Vitamin D and rheumatoid arthritis: Epidemiological and clinical studies. Clinical Reviews in Allergy & Immunology, 38(2–3), 111–119.
-
Prasad, A. S. (2008). Zinc in human health: Effect of zinc on immune cells. Molecular Medicine, 14(5–6), 353–357.
-
Nielsen, F. H. (2018). Magnesium, inflammation, and obesity in chronic disease. Nutrition Reviews, 76(6), 359–370.
-
Koseoglu, E., et al. (2021). Selenium supplementation in rheumatoid arthritis: Effect on disease activity and biomarkers. Biological Trace Element Research, 199(3), 1166–1173.
Other articles you may enjoy:
-
“Microbiome, Mitochondria, and Chronic Inflammation: The Hidden Trifecta”
-
“The Role of Luteolin in Combating Chronic Inflammation and Neurodegeneration”
Note: Always consult with a healthcare professional before considering any treatment options or significant dietary changes.